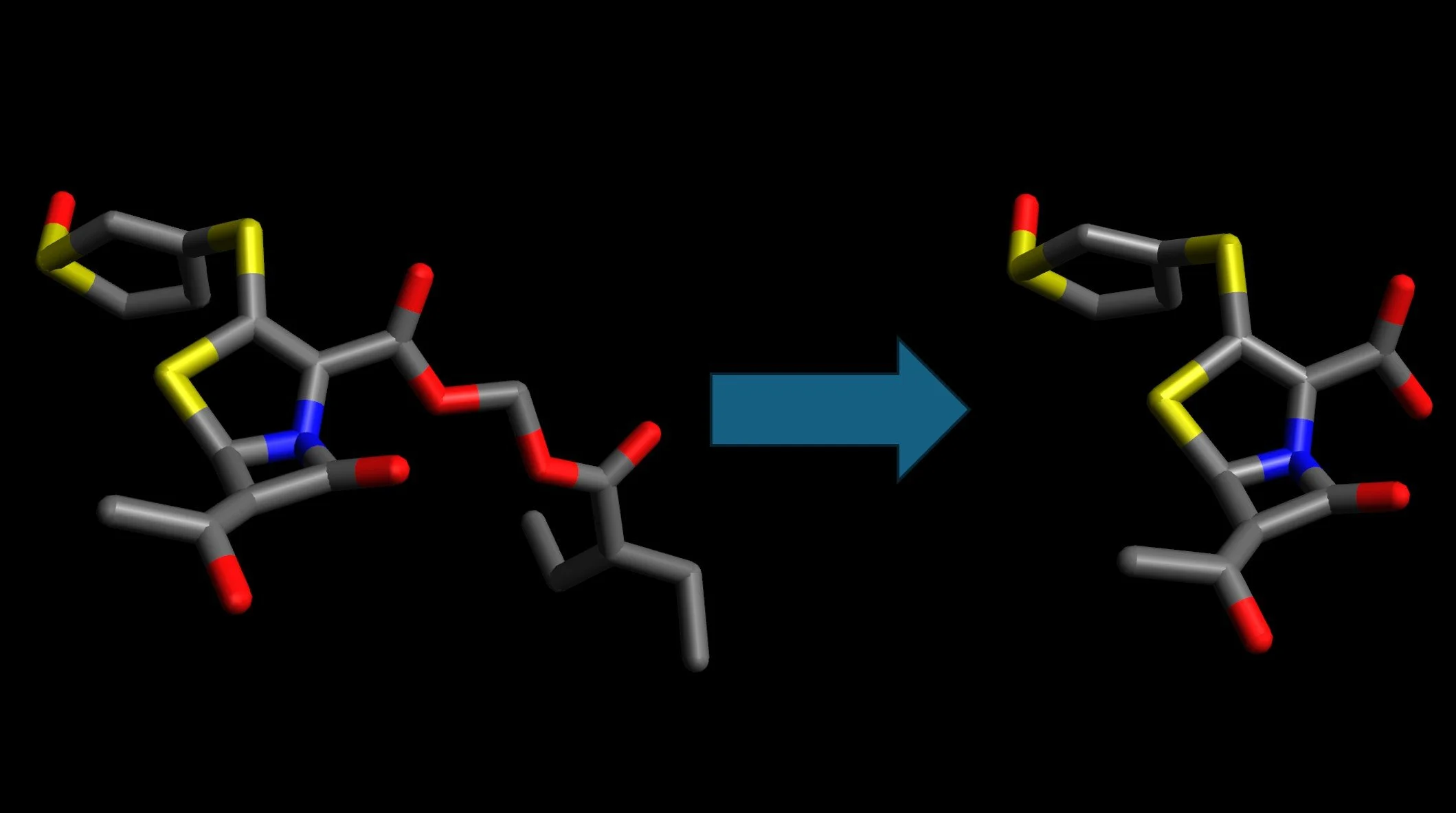
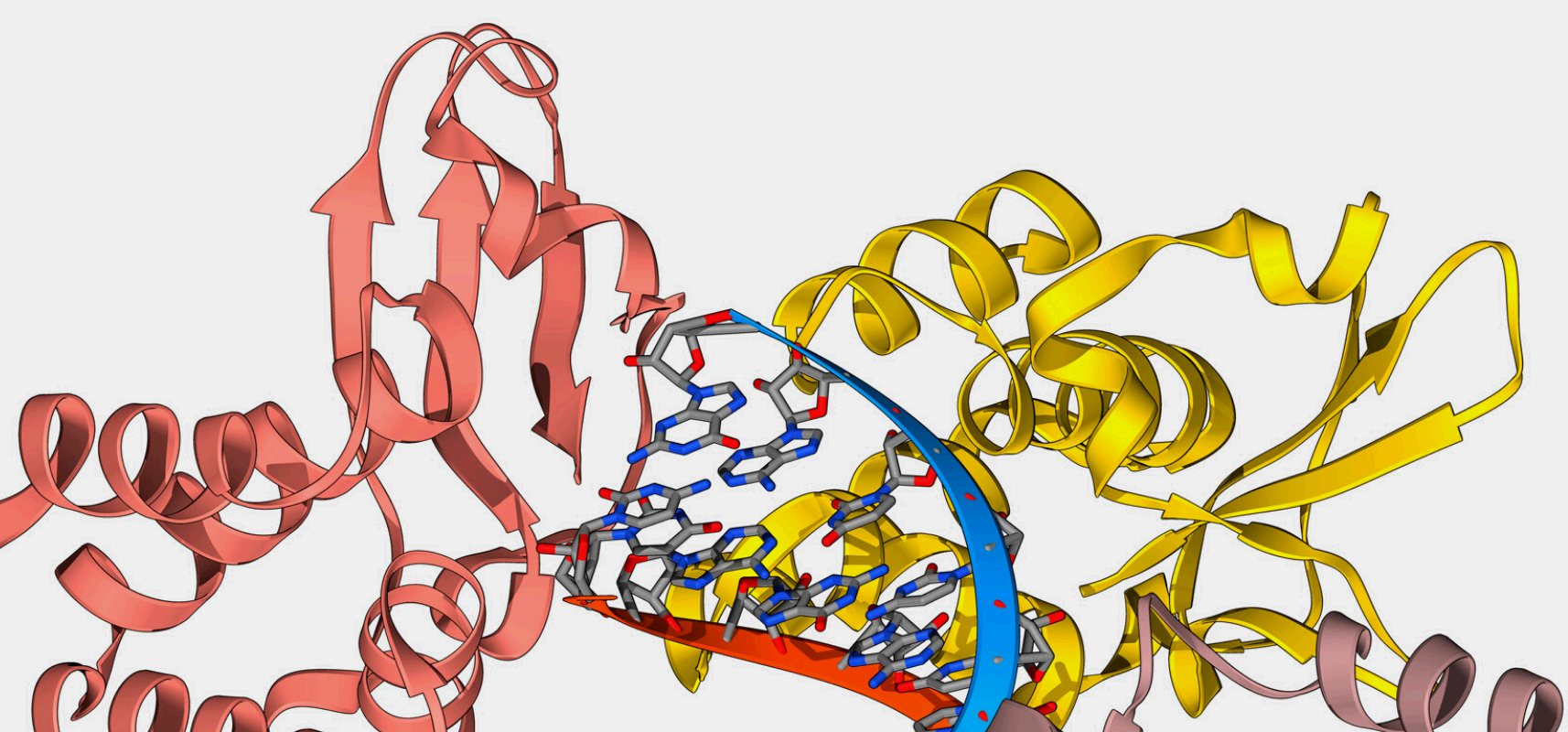
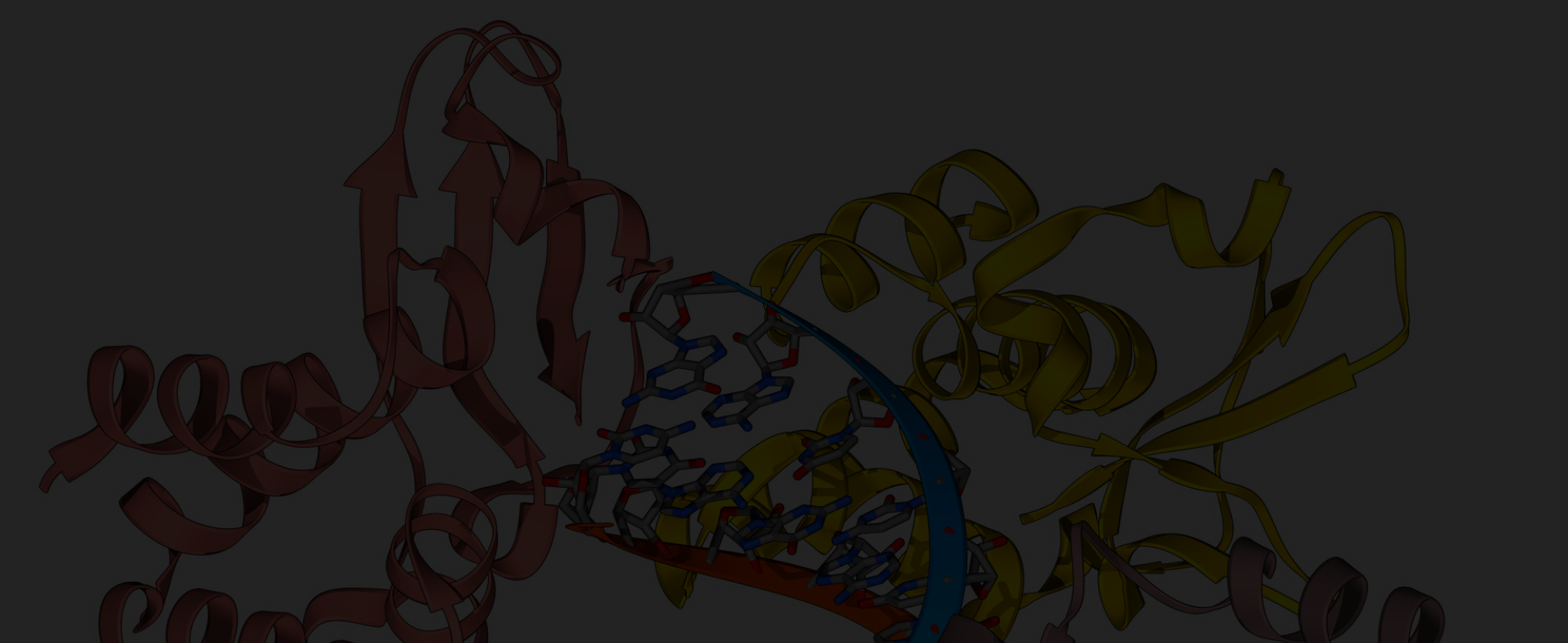
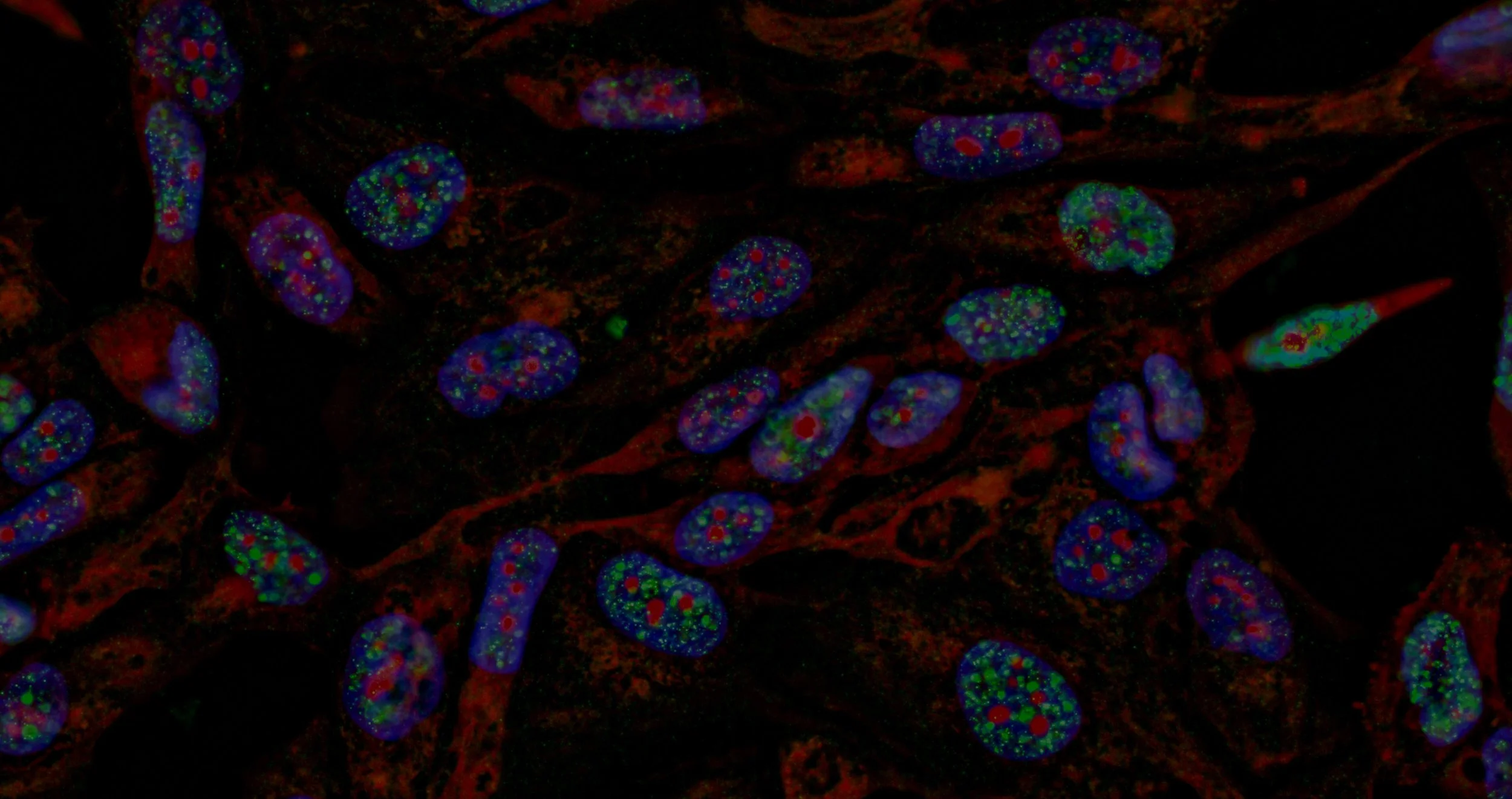
Crystal structure of JAK2 JH2 domain bound to JAK064
(PDB 7JYO).
BioPharmaWorks scientists are exploring the structural basis for small molecule interactions with proteins to improve their potency and devise new strategies for modulating protein function.
Talk to us to see what BioPharmaWorks can do for your project!
Crystal structure of JAK2 JH2 domain bound to JAK064
(PDB 7JYO).
BioPharmaWorks scientists are exploring the structural basis for small molecule interactions with proteins to improve their potency and devise new strategies for modulating protein function.
Talk to us to see what BioPharmaWorks can do for your project!
BioPharmaWorks: a preferred partner in drug discovery
Headquartered in Southeastern Connecticut, BioPharmaWorks brings deep experience and broad capabilities to pharmaceutical and biotechnology companies.
We collaborate with our clients, providing innovative solutions to address unmet medical needs. BioPharmaWorks delivers comprehensive support, whether it be through advising on specific projects, scientific advisory board membership, due diligence assessment, patent preparation, or driving design and synthesis of medicines through our CRO network. We operate at both ends of the discovery continuum - from establishing screening sequences and hit identification & characterization strategies that identify sound chemical equity to lead optimization delivering high quality clinical candidates.
For startup companies, we manage an extensive CRO network, applying our specialized expertise to advance your program through value inflections important for fundraising. For companies seeking to partner their assets, we offer advice on building a data package that is compelling to large pharmaceutical companies. We also offer due diligence services for companies seeking third party assessment of assets.
With over 300 years of combined experience in the pharmaceutical industry, let us work for you to deliver innovative medicines that transform clinical care.
Consulting Services and Drug Development:
MEDICINAL CHEMISTRY
Medicinal chemistry is at the heart of our effort with most clients. Based on our client’s objectives, we define a drug discovery screening funnel for efficient program progression. Clients often bring screening results and lead compounds to begin the journey. We provide a thorough literature review and use predictive tools to optimize activity and medicinal properties. Often the biological target is the only starting point, and we identify novel IP to develop into drug candidates. Working with CROs having broad capabilities, we manage compound synthesis, physicochemical property assessments and ADME assays to create a database of compound characteristics. We work directly with clients as compounds are optimized for development
PRODRUG DESIGN
Frequently, issues with drug exposure at specific tissues necessary for therapeutic action are due to suboptimal ADME properties. One strategy is to develop a prodrug of the active compound. The prodrug is absorbed intact and then is rapidly converted into the active agent that reaches the therapeutic target. Our extensive experience with prodrugs allows us to rapidly assess options to improve how a medicine is absorbed and distributed. Prodrugs can improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract - or help avoid drug interactions with cells or processes that are not the intended target. Over 15% of currently available medicines are prodrugs, making this expertise a key facet to many projects we encounter in our work.
STRUCTURAL BIOLOGY AND BIOPHYSICS
We use our extensive experience in structural biology and biophysics to help clients with fragment screening, hit validation and compound characterization. We work with a global network of CROs to execute structure projects for clients. Whether for a novel target or one that has been solved before, we work with the clients and the CRO to design constructs, apply appropriate purification and crystallization strategies, and obtain structures. We also apply our knowledge of structure-based drug design to help clients guide their chemistry design. Our scientists have extensive experience with establishing biophysical screening strategies and assay design to support hit characterization and validation. In addition, we provide guidance for the successful execution of biophysical assays such as NMR, SPR, MST or TSA and interpretation of data from these studies.
INDICATIONS DISCOVERY AND SYSTEMS BIOLOGY
We use our extensive experience in pathway analysis to validate molecular targets of interest to our clients, using various proprietary and public resources. We research MOA to discover new indications for novel or existing client compounds and to uncover possible adverse effects of leads at an early stage. We identify appropriate CROs, suggest assays for efficacy and toxicity, and provide expertise in interpreting the results. We can analyze drug targets plus preclinical and clinical data of small molecules and biologics in the portfolios of companies and academic institutes. Together with other colleagues in the company, we conduct patent search and analysis to establish patentability, residual patent life and potential commercial value of client projects.
NEUROPHARMACOLOGY
Our combined experience in discovering and developing medicines for Central Nervous System (CNS) disorders makes BPW an ideal partner for overseeing CNS projects. We provide a team of experts with deep knowledge of issues specifically associated with CNS drug development. We provide specialists in chemistry, ADME, preclinical models, in vivo microdialysis and clinical statistics. In addition, we work with appropriate CROs to support projects and move them forward.
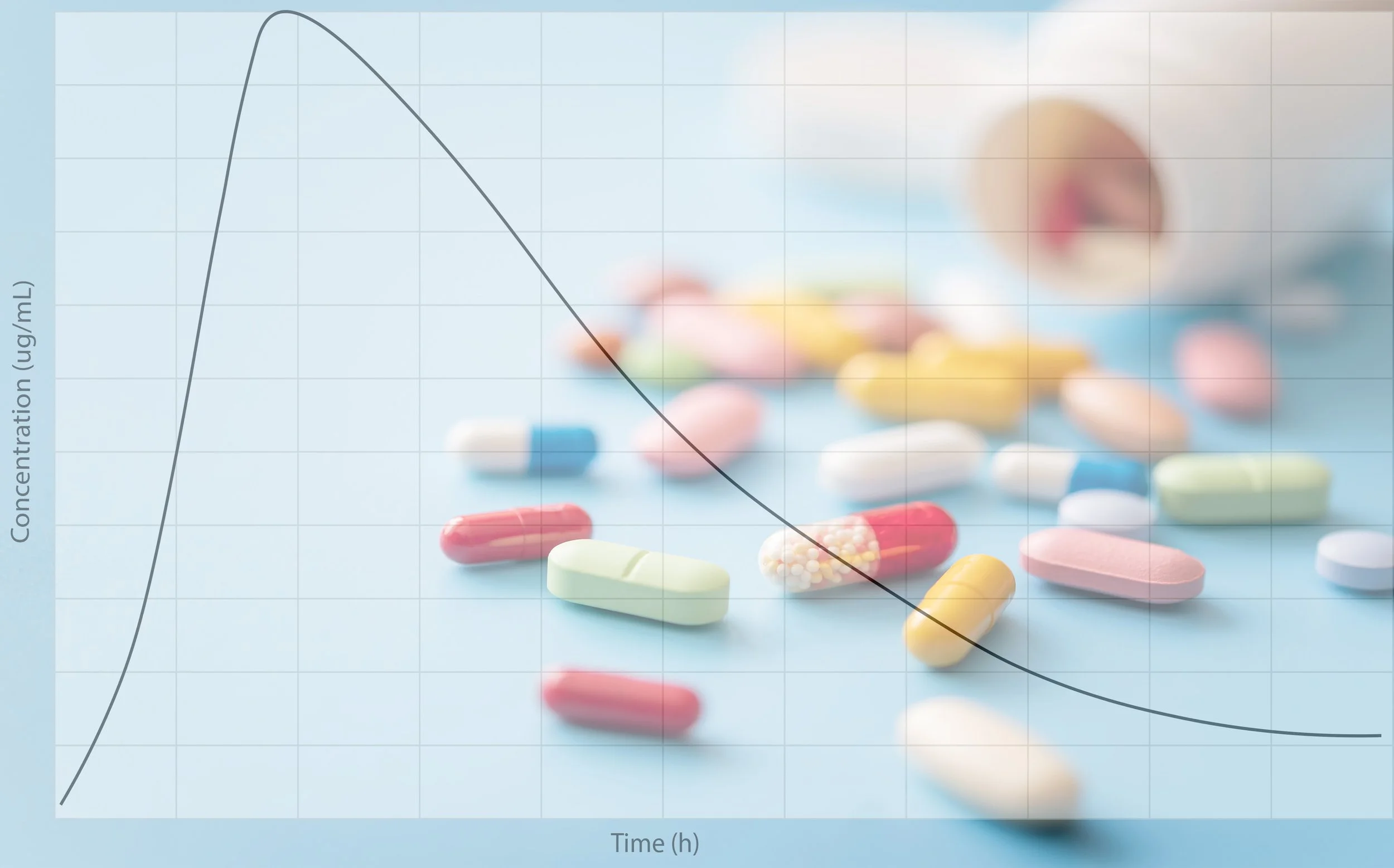
ADME AND PHARMACOKINETICS
We apply our experience in pharmacokinetics and drug metabolism to support our clients’ discovery programs. This involves defining project objectives enabling identification of NCEs having the appropriate predicted human efficacy and pharmacokinetic properties. We work with a network of CROs to assess the absorption, distribution, metabolism and excretion characteristics of lead compounds. This information is used to guide medicinal chemistry efforts producing clinical candidates.
CMC, CHEMICAL PROCESS RESEARCH AND DEVELOPMENT
BioPharmaWorks scientists can provide comprehensive or focused process chemistry, formulation and CMC support. We provide scientific oversight for the development of API synthesis and develop strategies for initial scale-ups (GMP or non-GMP) with appropriate CROs. We can design new processes suitable for scaleup as necessary. Beyond chemical synthesis, we can help identify stable solid forms and appropriate formulations to achieve your desired route of administration and pharmacokinetic profile. We have high level knowledge of drug product strategies such as spray dried dispersions, hot melt extrusion, capsules and IV formulations. We also support polymorph screening efforts to identify the solid form landscape and shore up your IP strategy.
BPW helps with the quality audits of the GMP vendors. We evaluate drug substance impurity profiles and set specifications at every stage of development. We track process-related genotoxic impurities and devise control strategies suitable for the drug substance and the client. In addition, we qualify impurities in tox studies.
Finally, BPW can write the CMC section for the IND and IMPD. We can oversee drug substance development and optimization for advanced clinical studies (Phase III) and commercial readiness, develop and implement DoE, PPQ and Validation strategies. We support clinical trials by ensuring appropriate flow of supplies to clinical sites in the US and elsewhere and provide Pharmaceutical Sciences risk assessment for potential licensing deals.
PRECLINICAL AND CLINICAL STATISTICS
We apply our expertise in pharmaceutical statistics to support projects from preclinical, through all clinical phases, including regulatory submission. Areas of support include design and analysis planning for preclinical and clinical studies, clinical development plans, analysis and interpretation of study data, and statistical evaluation of interim safety and efficacy results. In addition we provide membership in independent data and safety monitoring boards (DSMBs) plus due diligence evaluation for investment and in-licensing decisions.
LEGAL AND INTELLECTUAL PROPERTY
BioPharmaWorks understands our clients’ variable needs to identify and protect their intellectual property and other associated legal issues. Whether the program be first in class, unraveling literature in a crowded area or dealing with regulatory issues, we have the experience to identify the strategy to move your project forward.
Our clients realize that legal costs can be among the largest expenses for supporting a new program. Arbitraging these substantial costs of securing IP, we access skilled database searchers, prepare technical documents that our clients can take to their own Patent Attorneys or use law firms we have relationships with to cost-effectively progress a research program. Whatever your goals are, we can provide the technical support to draft documents that enable valuable IP.
BioPharmaWorks: a preferred partner in drug discovery
Headquartered in Southeastern Connecticut, BioPharmaWorks brings deep experience and broad capabilities to companies across the pharmaceutical and biotechnology industries.
We work as collaborators with our partners, providing innovative solutions to address unmet medical needs. BioPharmaWorks delivers comprehensive support, whether it be through advising on specific projects, scientific advisory board membership, due diligence assessment, patent preparation, or driving design and synthesis of medicines through our CRO network. We operate at both ends of the discovery continuum - from establishing screening sequences and hit identification & characterization strategies that identify sound chemical equity to lead optimization delivering high quality clinical candidates.
For startup companies, we manage an extensive CRO network, applying our highly specialized expertise to advance your program through value inflections important for fundraising. For companies seeking to partner their assets, we offer advice on building a data package that is compelling to large pharmaceutical companies. We also offer due diligence services for companies seeking to acquire assets and do a third party data assessment.
With over 300 years of combined experience in the pharmaceutical industry, let us work for you to deliver innovative medicines that transform clinical care.
Consulting Services and Drug Development
MEDICINAL CHEMISTRY
We can provide services from periodic design or synthesis consultations with your scientists to completely managing lead optimization for your program with our integrated network of CROs. Whichever partnership model you choose, our focus is on rapid delivery of high quality clinical candidates.
PRODRUG DESIGN
Frequently, issues with drug exposure at the site of action is due to suboptimal ADME properties. One strategy is to develop a prodrug of the active ingredient. The prodrug is absorbed intact and then is rapidly converted into the active agent that reaches the therapeutic target. Over 15% of currently available medicines are prodrugs, making our expertise a key asset to many projects.
STRUCTURAL BIOLOGY AND BIOPHYSICS
We use our extensive experience in structural biology and biophysics to rapidly enable structure based design. We work with CROs to design constructs, apply appropriate purification and crystallization strategies, and obtain novel structures of their protein targets bound to lead molecules. We also guide the successful execution and interpretation of biophysical assays such as NMR, SPR, MST or TSA supporting fragment screening campaigns and hit validation studies.
INDICATIONS DISCOVERY AND SYSTEMS BIOLOGY
We use our extensive experience in pathway analysis, integrating preclinical and clinical data to understand potential safety and efficacy attributes for validating disease targets. We research MOA to discover new indications for novel or existing client compounds as well as to uncover possible adverse effects of leads at an early stage. We identify appropriate CROs, suggest assays for efficacy and toxicity, and provide expertise in interpreting the results.
NEUROPHARMACOLOGY
Our combined experience in discovering and developing drugs for Central Nervous System (CNS) disorders makes BPW an ideal partner in this disease area. We provide a team of experts with deep knowledge and understanding of issues specifically associated with CNS drug development. In addition, we work with appropriate CROs to support projects and move them forward.
ADME AND DMPK
We apply our extensive experience in pharmacokinetics and drug metabolism to support our clients’ discovery programs. We help define near and long-term project objectives to help you deliver candidates with the required predicted human efficacy and pharmacokinetic properties. We work with a network of CROs able to assess the absorption, distribution, metabolism and excretion characteristics of compounds of interest. We then optimize compound properties for a particular indication.
CMC, CHEMICAL PROCESS RESEARCH AND DEVELOPMENT
We can provide comprehensive or focused process chemistry, formulation and CMC support. Our capabilities include scientific oversight for the development of API synthesis, executing GMP or non-GMP scaleup with appropriate CROs and designing new scalable processes. Beyond chemical synthesis, we can help identify stable solid forms and appropriate formulations to achieve your desired route of administration and pharmacokinetic profile. We have strong expertise in polymorph screening, spray dried dispersions, hot melt extrusion, capsules and IV formulations. Finally, BPW helps with the quality audits of the GMP vendors and can write the CMC section for the IND and IMPD.
PRECLINICAL AND CLINICAL STATISTICS
We apply our expertise in statistics to provide support to projects from preclinical through all clinical phases, including regulatory submission. Areas of support include design and analysis planning for preclinical and clinical studies, clinical development plans, analysis and interpretation of study data, and statistical evaluation of interim safety and efficacy results. In addition we provide membership in independent data and safety monitoring boards (DSMBs) plus due diligence evaluation for investment and in-licensing decisions.
LEGAL AND INTELLECTUAL PROPERTY
BioPharmaWorks understands our clients’ needs to protect their intellectual property and other associated legal issues. Whether your program is first in class, unraveling literature in a crowded area or dealing with regulatory issues, we have the experience to identify the strategy to move your project forward.
Our clients realize that legal costs can be among the largest expenses for supporting a new program. Arbitraging these substantial costs of securing IP, we access skilled database searchers, prepare technical documents that our clients can take to their own Patent Attorneys or use law firms we have relationships with to cost-effectively progress a research program.